Low Alloy Steel Ball Mill Liners Design
The main function of the ball mill liner is to protect the mill and use the convex peak of the liner to play the ball to grind and crush the material. Therefore, the main failure mode of the liner is abrasive wear under the repeated impact of small energy. Under the condition of abrasive wear, wear resistance directly affects the service life of parts, so the research on wear resistance is also an important technical problem. This project is put forward for the failure of liner under abrasive wear conditions, and the purpose is to improve the comprehensive performance of low alloy steel wear-resistant material under this condition.
Low Alloy Steel Ball Mill Liners Material Analysis
Wear-resistant low alloy steel materials usually contain alloying elements such as silicon, manganese, chromium, molybdenum, nickel, etc. The strong influence of these alloying elements on the matrix structure and hardenability of the material can be brought into full play, which can make the material have better wear resistance.
Carbon: Carbon is an important element that affects the strength, hardness, toughness, hardenability, and wear resistance of cast steel. If the carbon content is too high, the hardness of the high carbon martensite formed after heat treatment is high, but the toughness is low, and cracks are easy to form during heat treatment; if the carbon content is too low, the hardenability and hardness of the casting are poor, and the wear resistance is poor. Considering the combination of hardness and toughness, two different carbon contents (mass fraction, the same below) were adopted in this material, which was 0.30% – 0.35% and 0.40% – 0.45%, respectively. The effects of two carbon contents on the microstructure and properties of low alloy steel were studied.
Chromium: Chromium is one of the basic elements of wear-resistant materials. Its main function is to improve the hardenability of steel, strengthen the matrix by solution, improve the oxidation resistance of steel and increase its corrosion resistance. Chromium and iron form continuous solid solution and form a variety of compounds with carbon. The complex carbide of chromium has a significant effect on the properties of steel, especially the improvement of wear resistance. Cr and Fe form intermetallic compound FeCr. Chromium can significantly increase the hardenability of steel, but it also tends to increase the temper brittleness of steel. Chromium improves the temper brittleness of the steel and reduces the martensite point ms of the steel. When chromium is added into pure iron and steel, the strength and hardness can be improved at a certain chromium content. Considering the effect of chromium on Microstructure and properties of steel, the content of chromium is 1.0% ~ 1.4%. The effect of chromium on Microstructure and properties of steel is observed by experiment.
Nickel: Nickel and carbon do not form carbides. They are the main alloying elements for forming and stabilizing austenite. In this respect, the role is second only to carbon and nitrogen. Nickel and iron exist in the α phase and γ phase of steel in the form of mutual solubility, which makes them strengthen. By refining the grain size of the α phase, the low-temperature properties, especially the toughness of steel are improved. Nickel can improve the hardenability of steel by reducing the critical transformation temperature and the diffusion rate of elements in steel. Some physical properties of steel and alloy can be significantly improved when nickel content is high. The effect of nickel on toughness, plasticity, and other process properties of steel is less than that of other alloy elements. In addition, as nickel is a rare element and an important strategic material, the nickel content is set at 0.4% based on the above factors.
Molybdenum: Molybdenum belongs to the element of the closed γ phase region. Molybdenum exists in the solid solution phase and carbide phase in steel. In the carbide phase, when the content of Mo is low, it forms composite cementite with iron and carbon; when the content is high, it forms its own special carbide. The effect of molybdenum in steel can be summarized as improving hardenability, improving thermal strength, preventing temper brittleness, increasing remanence and coercivity, improving the corrosion resistance of alloy in some media and preventing pitting corrosion tendency. Molybdenum has a solid solution strengthening effect on ferrite and improves the stability of carbides, so it has a favorable effect on the strength of steel. The effect of molybdenum on the Temper Embrittlement of steel is quite complicated. As a single alloy element, Mo increases the temper brittleness of steel, but when it coexists with other elements, such as chromium and manganese, molybdenum reduces or suppresses the temper brittleness caused by other elements. Because the different content of molybdenum may have different effects on the properties of steel, we decided to select the content of molybdenum in the experiment as 0.25% – 0.35% and 0.45% – 0.60%.
Manganese: Manganese is a good deoxidizer and desulfurized. Manganese and iron form solid solution, which improves the hardness and strength of ferrite and austenite in steel; at the same time, it is a carbide forming element, which enters cementite to replace some iron atoms. Manganese can refine pearlite and improve the strength of pearlite steel indirectly by reducing the critical transformation temperature. Manganese can also significantly reduce the AR1 temperature and the austenite decomposition rate of steel. Manganese has a significant effect on improving the strength of low and medium carbon pearlite steels. However, as an alloying element, manganese has its disadvantages. When the content of Mn is higher, the grain size of the steel tends to be coarsened and the sensitivity of temper brittleness is increased. It is easy to produce white spots in steel due to improper cooling after smelting, casting, and forging. Considering the effects of manganese on the microstructure and properties of steel, the content of manganese is 1.1% ~ 1.4%.
Silicon: Silicon is one of the common elements of steel. As an alloying element, the content of silicon in steel should not be less than 0.40%. Silicon does not form carbide in steel, but exists in ferrite or austenite in the form of solid solution. It improves the strength of the solid solution in steel, and its cold work deformation hardening rate is very strong, second only to phosphorus, but also reduces the toughness and plasticity of steel to a certain extent. If the content of silicon is more than 3%, the plasticity, toughness, and ductility of the steel will be significantly reduced. Silicon can improve the elastic limit, yield limit, yield ratio, fatigue strength, and fatigue ratio of steel. Silicon can increase the annealing, normalizing, and quenching temperatures of steel, reduce the diffusion rate of carbon in ferrite, and increase the tempering stability of steel. Considering the effects of silicon on properties and microstructure of steel, the content range of silicon is 1.1% ~ 1.4%.
Rare earth: There are two main functions of rare earth in steel, one is purification and the other is alloying. Re can improve as-cast microstructure, refine grain size, purify molten steel, modify non-metallic inclusions, improve their morphology and distribution, and play a role in microalloying. Improve toughness and casting properties (hot cracking resistance and fluidity), improve strength. However, due to the uncertainty of adding method and amount, if the rare earth content is too much, it may have an adverse effect on the properties of steel. Therefore, the content of rare earth in this material is determined to be 0.04% – 0.06%.
Boron: The outstanding function of boron in steel is that the hardenability of steel can be increased by a small amount of boron (0.001%). When the content of boron is more than 0.007%, it will lead to hot embrittlement of steel. Therefore, the boron content in this material is determined to be 0.003%.
The main elements of the experimental materials were selected according to the above analysis. The carbon content of sample #1 and #2 is 0.30% – 0.35%, and the content of molybdenum is 0.25% – 0.35%; the carbon content of sample #3 and #4 is 0.40% – 0.45%, and the molybdenum content is 0.45% – 0.60%.
Low Alloy Steel Ball Mill Liners Casting Process
In this experiment, a 50 kW medium frequency induction furnace is used for smelting. In order to reduce the oxidation of the furnace charge, the stirring of molten metal should be avoided as far as possible. In the later stage of smelting, the feeding block should not be too large and should be dried to a certain temperature to prevent splashing at the furnace’s mouth. The feeding sequence is scrap steel, pig iron → nickel plate, ferrochrome, ferromolybdenum → ferrosilicon, ferromanganese → rare earth ferrosilicon, and finally adding aluminum for deoxidation.
After dry mixing for 2-3 min, the molding sand was mixed with water and glass for 4-6 min. After the mold is made, the mold is hardened by blowing carbon dioxide (blowing pressure is 0.15-0.25 MPa, blowing time is 1-2 min). Before pouring, the sand mold and alloy are preheated in the furnace and kept dry. The preheating temperature is about 100 ℃.
Low Alloy Steel Ball Mill Liners Heat Treatment
The properties of as-cast materials must be properly heat treated. In the actual working condition, the martensite structure with high hardness, high strength, and good toughness should be obtained, and the heat treatment process of quenching and tempering is adopted. The undercooled austenite of low alloy wear-resistant steel is relatively stable, and the cooling rate of oil in the low-temperature zone is much smaller than that of water, so oil is the most suitable quenching medium. Tempering is to reduce or eliminate the residual stress caused by quenching, improve the plasticity and toughness of the material, reduce its brittleness, and obtain the appropriate combination of plasticity, toughness, and hardness. Therefore, the quenching temperatures of 850, 880, 910, and 930 ℃ are selected for 1 h. The tempering temperature is 200, 230, 260, and 290 ℃, and the holding time is 2 h.
Low Alloy Steel Ball Mill Liners Performance Testing
The hardness of the samples was measured by the hr-150 Rockwell hardness tester, and the microstructure was observed by an Olympus BH-2 metallographic microscope.
| Tab.1 Hardness of the samples as-cast(HRC) | ||||
| Sample | First Point | Second Point | Third Point | Avg. |
| #1 | 31 | 36 | 35 | 34 |
| #2 | 31 | 35.5 | 37 | 34.5 |
| #3 | 38 | 39 | 40 | 39 |
| #4 | 39 | 38.5 | 41 | 39.5 |
It can be seen from Table 1 that the hardness values of samples #1 and #2 are almost the same, but with the increase of carbon content, the hardness of samples #3 and #4 increases obviously.
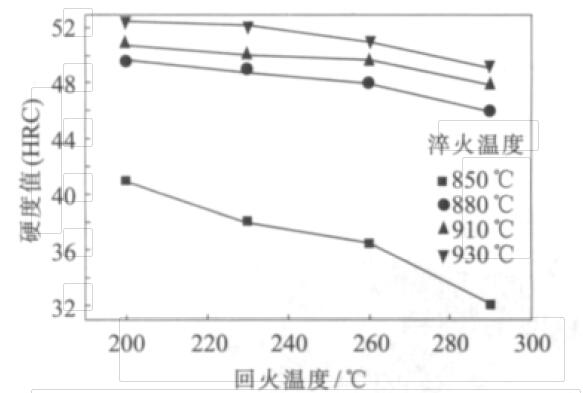
Fig.1 Hardness of No.1 sample at different heat treatment temperature
It can be seen from Fig. 1 that, on each quenching temperature curve, with the increase of tempering temperature, the hardness value of #1 sample basically shows a downward trend, but the decrease range is not very large, and the downward trend is relatively gentle; on the impact toughness curve, with the increase of quenching temperature, the value decreases, but with the increase of tempering temperature, its value increases. With the increase of tempering temperature, the carbon content, alloying element content, dislocation density, and twinning number in the martensite matrix decrease, so the amount of strengthening also decreases, so the hardness decreases. With the increase of tempering temperature, the matrix recrystallization and carbide point coarsening and spheroidizing. Because the carbide spheroidization reduces the dislocation slip distance and makes the slip distance shorter, the dislocation can not cut them, so the toughness shows an upward trend.
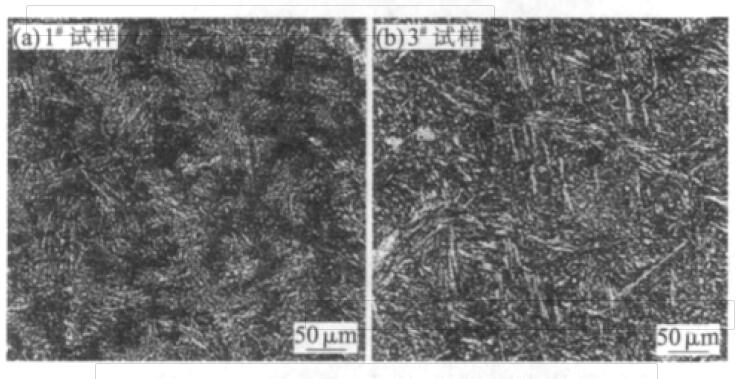
Fig.2 Microstructure of as-cast sample No.#1 and #3
It can be seen from Fig. 2 that the microstructure of samples #1 and #3 is pearlite。
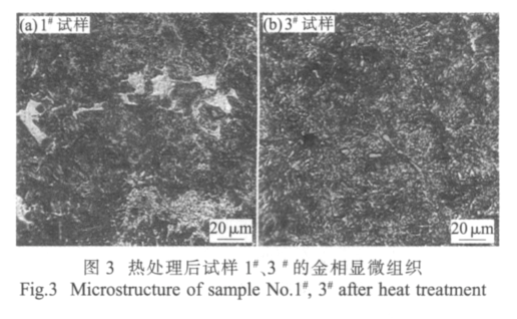
Fig.3 Microstructure of sample No.#1 and #3 after heat treatment
Figure 3 shows the metallographic structure of the sample after quenching at 910 ℃ and tempering at 230 ℃. It can be seen that the microstructure and matrix of the two kinds of samples are lath martensite. The microstructure of the sample is uniform and the grain size is fine.
| Tab.2 Results of wearing experiment after heat treatment | |||||
| Sample | First Lose weight w/g | Lose weight w/g | Avg. Lose weight w/g | Hardness (HRC) | Wear resistance |
| #1 | 0.04013 | 0.03705 | 0.03859 | 50 | 25.91345 |
| #2 | 0.03874 | 0.03615 | 0.03744 | 51.3 | 26.7094 |
| #3 | 0.03091 | 0.03461 | 0.03276 | 53.6 | 30.52503 |
| #4 | 0.03288 | 0.0245 | 0.02869 | 55.5 | 34.85535 |
It can be seen from Table 2 that with the increase of hardness, the wear resistance of #1 – #4 samples increases in turn. Therefore, it can be concluded that the wear loss of materials is directly related to the hardness of materials. The higher the hardness is, the smaller the weight loss is, the better the wear resistance of materials is. In addition, the dispersed carbides in the matrix also contribute to the wear resistance of the materials, but the effect is less than that of the hardness because of the few carbides precipitated.
Results
- The low alloy steel ball mill liners studied in this paper have high hardenability and high tempering stability.
- After quenching at 850-930 ℃ and tempering at 200-290 ℃, finely tempered lath martensite is obtained, which makes the steel have high strength, high toughness and high wear resistance.
- The higher the hardness, the smaller the weight loss, the better the wear resistance.
About the author:
China Mill liners manufacturer, Qiming Machinery is a leader in the design, manufacture, and supply of mill liners for mineral processing and quarrying industries. It offers customers complete wear liner solutions for mills that increase performance, equipment availability, and lower maintenance costs. It’s mill liners are also tested to withstand the acidity level of different elements that may be present in the milling process. Longer milling life to your machine means fewer expenses and more profit or income to your company.

 Download Brochure
Download Brochure Product List
Product List